Science
Montreal’s AI Surgical Device Extends Lives and Offers Hope
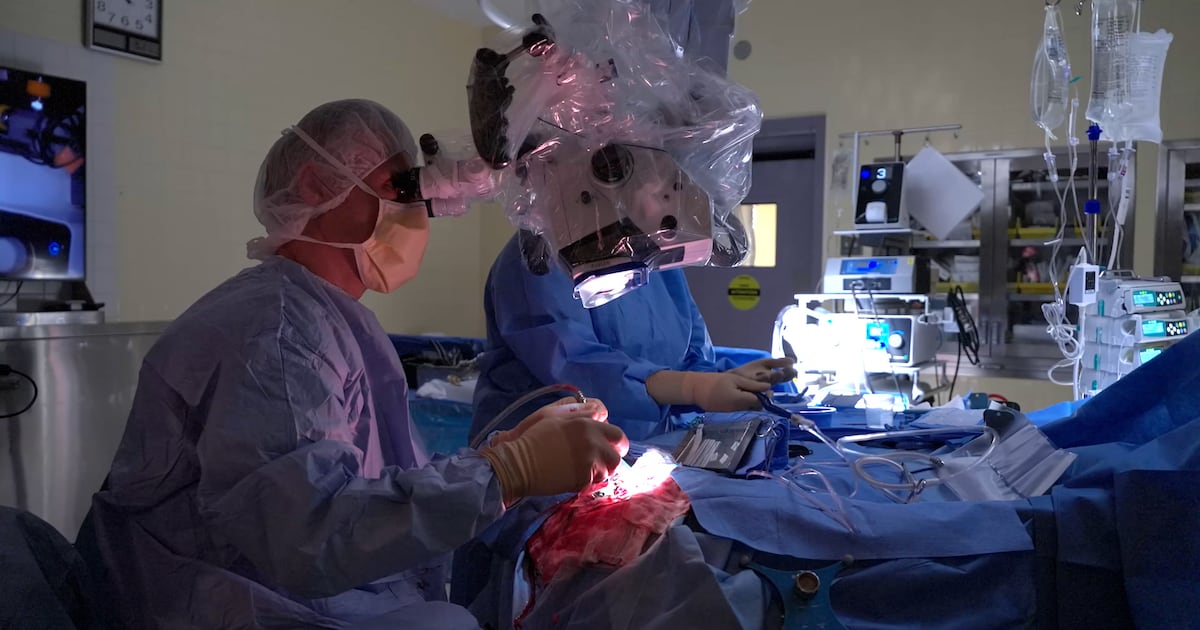
A groundbreaking surgical device developed in Montreal is transforming cancer treatment by utilizing artificial intelligence to enhance surgical precision and extend patients’ lives. This innovation, known as SENTRY, has already made a significant impact on individuals facing dire diagnoses, including Peter Ross, who was given just 14 months to live after a brain tumor diagnosis nearly four years ago.
Ross’s journey took a remarkable turn when he was treated by Dr. Kevin Petrecca, a neurosurgeon at Montreal Neuro and co-founder of SENTRY. Thanks to the device, Ross is now preparing to welcome his first grandchild, a milestone he attributes directly to the advanced surgical technology. “It’s given me the rest of my life,” Ross expressed, reflecting on his unexpected recovery. His wife, Sandrine Menard, echoed his sentiments, stating, “Whatever happens, we’re grateful.”
SENTRY is a handheld device designed to assist surgeons in distinguishing between cancerous and healthy tissue during surgery. According to Dr. Petrecca, the device boasts an impressive accuracy rate, identifying tumors correctly 98.7 percent of the time. Moreover, it guarantees a 100 percent accuracy rate for identifying normal brain tissue. This level of precision is vital, as cancerous cells are often invisible to the naked eye and undetectable by traditional technologies.
The functionality of SENTRY is both rapid and efficient. Surgeons receive feedback within three seconds of contact with the tissue, providing immediate insight into whether tumor cells are present. This capability enables surgeons to excise more cancerous tissue than ever before, significantly improving patient outcomes. Dr. Petrecca noted that the use of SENTRY can potentially extend a patient’s life by two to five times longer than initially expected.
While SENTRY has predominantly been tested in brain surgeries, its application is not limited to this area. The device has also shown promise in detecting cancer in other regions, including the breast and lungs. Hundreds of patients have participated in clinical trials involving SENTRY, which have yielded encouraging results.
Reflecting on his experience, Ross stated, “I take every extra day that’s given to me to go out and walk and just enjoy life because I owe that to this surgery.” As the device moves closer to widespread availability, Ross hopes it will help many more patients reach their own significant life milestones.
The next crucial step for SENTRY is obtaining FDA approval, with a pivotal trial scheduled for May 2024. This milestone is critical for the device’s future and could pave the way for broader adoption in medical settings. The ongoing advancements in surgical technology like SENTRY highlight a significant shift in cancer treatment, offering renewed hope to patients and their families.
-

 Politics4 weeks ago
Politics4 weeks agoSecwepemc First Nation Seeks Aboriginal Title Over Kamloops Area
-

 World5 months ago
World5 months agoScientists Unearth Ancient Antarctic Ice to Unlock Climate Secrets
-

 Entertainment5 months ago
Entertainment5 months agoTrump and McCormick to Announce $70 Billion Energy Investments
-

 Science5 months ago
Science5 months agoFour Astronauts Return to Earth After International Space Station Mission
-

 Lifestyle5 months ago
Lifestyle5 months agoTransLink Launches Food Truck Program to Boost Revenue in Vancouver
-

 Technology3 months ago
Technology3 months agoApple Notes Enhances Functionality with Markdown Support in macOS 26
-

 Lifestyle3 months ago
Lifestyle3 months agoManitoba’s Burger Champion Shines Again Amid Dining Innovations
-

 Top Stories2 months ago
Top Stories2 months agoUrgent Update: Fatal Crash on Highway 99 Claims Life of Pitt Meadows Man
-

 Politics4 months ago
Politics4 months agoUkrainian Tennis Star Elina Svitolina Faces Death Threats Online
-

 Sports5 months ago
Sports5 months agoSearch Underway for Missing Hunter Amid Hokkaido Bear Emergency
-

 Politics5 months ago
Politics5 months agoCarney Engages First Nations Leaders at Development Law Summit
-

 Technology5 months ago
Technology5 months agoFrosthaven Launches Early Access on July 31, 2025




















