A groundbreaking study has unveiled a detailed atlas illustrating how pesticides affect gut bacteria, offering insights into potential probiotic interventions. This research, conducted by a team at The Ohio State University and published in Nature Communications, marks the first comprehensive mapping of changes to specific gut bacteria due to interactions with pesticides in both laboratory and animal models.
Researchers discovered that over a dozen pesticides influence the growth patterns of human gut bacteria, affecting nutrient processing and accumulating within certain bacteria. This atlas of molecular mechanisms, now publicly available, is poised to become a crucial resource for targeted studies on diseases and therapeutic strategies.
Understanding Pesticide-Gut Microbe Interactions
According to the study, experiments in mice revealed that one species of gut bacteria could offer protection against pesticide toxicity, suggesting a probiotic approach to mitigating some harmful health effects, such as inflammation. “We’ve provided further understanding of how pesticides or environmental pollutants impact human health by modulating an important collection of microorganisms,” stated Jiangjiang Zhu, the study’s senior author and associate professor of human sciences at The Ohio State University.
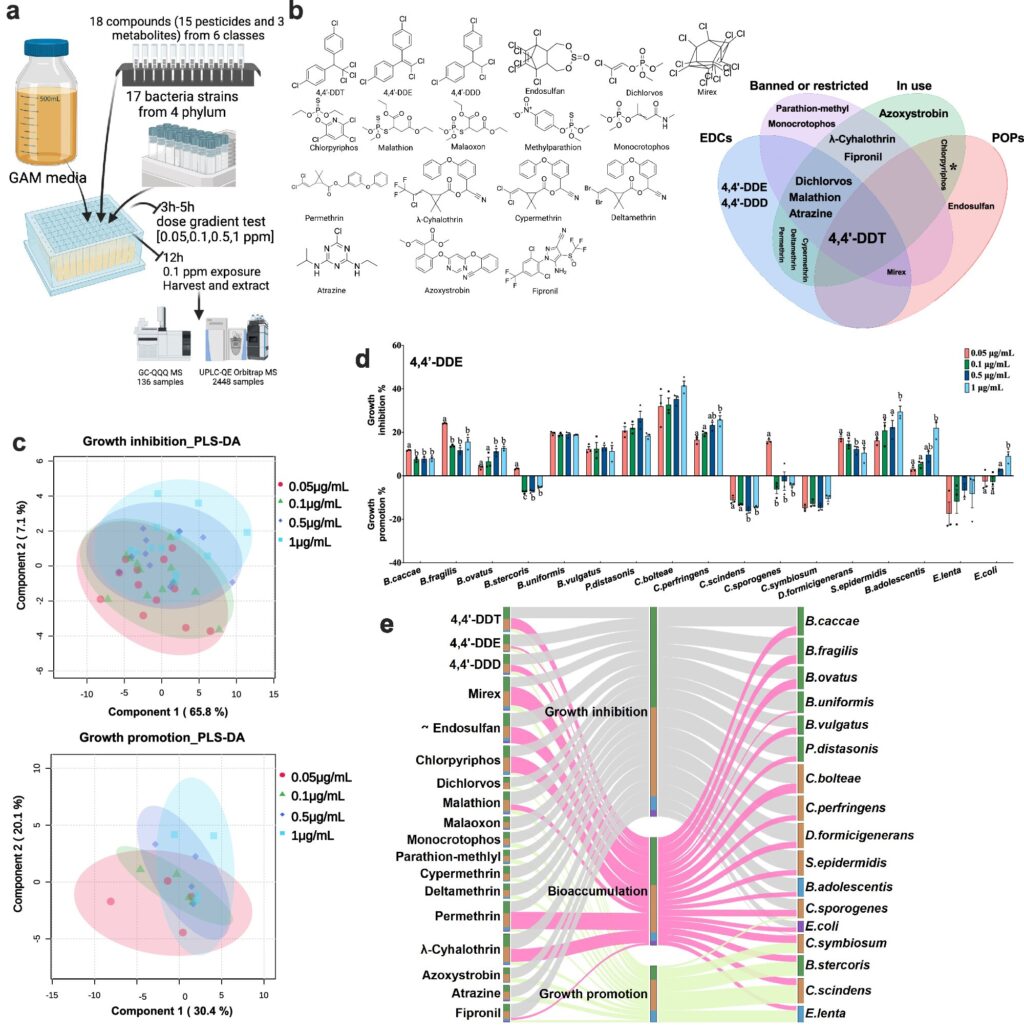
The research involved interactions between 18 pesticide compounds, chosen for their widespread agricultural use, and 17 species from four major bacterial domains in the human gut. These bacteria are linked to either health maintenance or disease states. Among the pesticides studied were DDT, atrazine, permethrin, and chlorpyrifos, some of which, despite usage restrictions, still linger in soil and water.
“We grew bacteria in culture and exposed them to relevant concentrations of pesticides to see how microbes responded to those pesticide exposures,” explained Li Chen, the study’s first author and senior research associate in Zhu’s lab.
Network Analysis and Metabolic Changes
The team developed a bacteria-pesticide interaction network, detailing which pesticides promoted or inhibited bacterial growth and which bacteria absorbed pesticide chemicals. This network highlights how pesticide exposure can prolong within the body, affecting metabolites across the gut microbiota.
Chen noted, “Most previous environmental health studies reported that pesticide contamination affects the overall composition of gut bacteria. We showed those pesticides really can affect specific gut bacteria and detailed how these changes will affect the general composition.”
The analysis identified specific metabolic changes in 306 pesticide-gut microbe pairs, examining how altered growth patterns and chemical accumulation affected metabolites. Metabolites, the molecular products of biochemical reactions, play numerous roles, from altering metabolic processes to signaling cell functions and immune system activation.
Implications for Human Health
The study also focused on lipids, essential compounds produced by gut microbes, which are critical to many body functions. Researchers observed the effects of pesticide exposure in healthy mice, first given antibiotics to clear their digestive systems of microbes, then introduced with Bacteroides ovatus, a common human gut bacterium.
Results confirmed that pesticides caused inflammation in multiple organs in mice, and the introduced bacteria after chemical exposure triggered various changes in metabolic activity and lipid production. Specifically, an increase in some lipid classes inhibited the signaling pathway of a protein linked to oxidative stress.
“We identified microbes that may modulate the toxic effect of pesticides to the host by somehow buffering the inflammation process,” Zhu said, emphasizing the potential for intervention strategies to prevent larger-scale damage.
Future Directions and Broader Implications
Looking ahead, Zhu’s lab plans to explore where metabolic changes to gut microbes fit into various health and disease conditions post-pesticide exposure. Zhu anticipates that other scientists will build on these findings, potentially leading to new disease research and intervention strategies.
“We are mapping out this central interaction between pesticides and gut microbes. Other labs can leverage what we have discovered—for example, after exposure to a pesticide, gut microbe reactions may lead to downstream consequences that contribute to disease research and eventually help with predicting targets or identifying an intervention strategy,” he said.
The study included contributions from researchers at Yale University, Zhejiang Academy in Hangzhou, China, and Johns Hopkins University, highlighting the collaborative effort in advancing our understanding of pesticide impacts on human health.
For more information, refer to the study by Li Chen et al, “Mapping pesticide-induced metabolic alterations in human gut bacteria,” published in Nature Communications.