Entertainment
Wellness Influencers Push Unapproved Peptide Injections Amid Risks
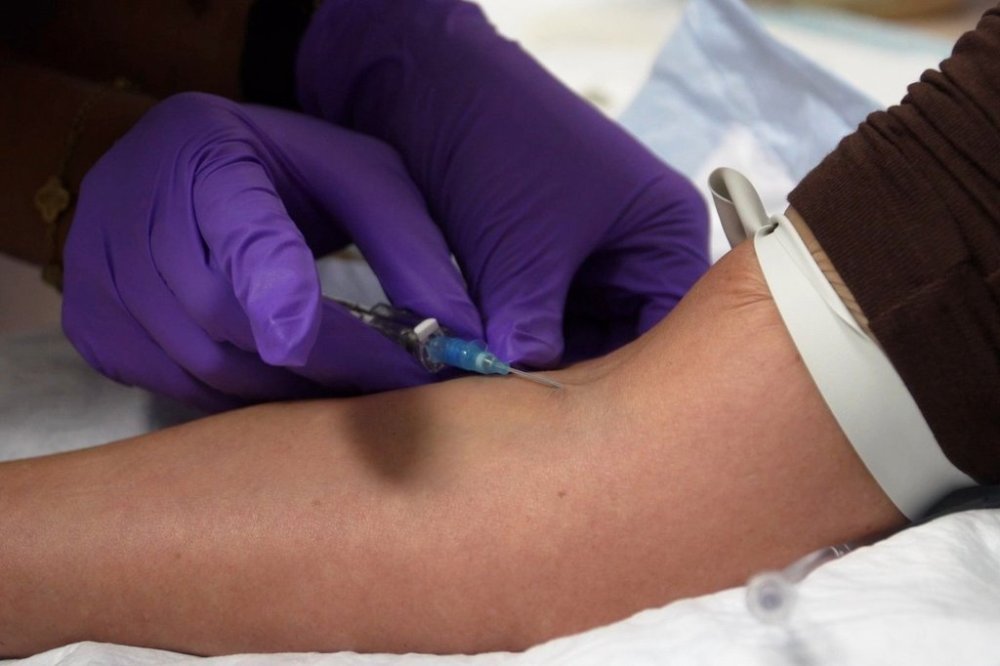
Unapproved peptide injections have surged in popularity, championed by wellness influencers, fitness coaches, and celebrities as a means to enhance muscle growth, promote weight loss, and achieve a youthful appearance. These products, which can cost between **$300 to $600** per injectable vial, are often marketed through online platforms and longevity clinics, where some charge membership fees reaching thousands of dollars monthly. Despite their appeal, many of these peptides lack extensive human studies, prompting concerns over potential allergic reactions, metabolic disturbances, and other serious side effects.
The human body utilizes peptides, which are short chains of amino acids, for vital functions. For instance, insulin regulates blood sugar levels and aids in energy production. Similarly, the approved weight loss drugs known as **GLP-1s** (glucagon-like peptides) are derived from hormones that help control blood sugar. While the **FDA** (Food and Drug Administration) has sanctioned these substances as safe and effective, numerous peptides have not received such approval, even though some have demonstrated promising results in animal studies.
Peptides have a long history of off-label prescriptions by healthcare providers for various conditions, including gastric ulcers and nerve disorders. Yet, in recent years, their use has gained traction among wellness advocates and public figures, leading to the promotion of lesser-known peptides for questionable benefits such as injury healing and skin enhancement. Notable examples include **BPC-157**, thymosin alpha, and GHK-Copper, with some being classified as banned doping substances by sports regulators.
Concerns are heightened by the trend of combining multiple peptides. Dr. **Eric Topol** of the **Scripps Research Translational Institute** expressed alarm over influencers advocating for combinations of up to four different peptides, calling it “dangerous.” The influence of celebrities has further amplified this trend. **Joe Rogan**, for example, has frequently discussed his use of **BPC-157** for injury recovery, while **Jennifer Aniston** has shared her experiences with peptide injections for skincare, actively endorsing a company that markets peptide-enriched supplements.
Kay Robins, a clinical nurse and operator of **Pure Alchemy Wellness** in **San Diego**, noted the increased mainstream interest in peptides due to celebrity endorsements. She has since ceased offering **BPC-157** and other peptides that the FDA has flagged for scrutiny.
Legally, many of the unapproved peptides sold online are classified as illegal substances. Any injected product intended for health benefits is considered a drug and requires FDA approval for sale. The FDA categorizes numerous peptides as biologics, necessitating stringent manufacturing and storage regulations. In recent years, the agency has identified over two dozen peptides that should not be produced by pharmacies due to safety risks.
Some companies attempt to sidestep regulations by marketing peptides as dietary supplements, particularly in pill or powder form. While these supplements are subject to less rigorous oversight than drugs, the FDA mandates that they contain only ingredients from an approved list, which most peptides do not belong to. Experts assert that orally consumed peptides are unlikely to be effective, as they typically dissolve in the digestive system.
Most injectable peptides available in the United States are produced by compounding pharmacies, which tailor medications not offered by manufacturers. These pharmacies are regulated at the state level and do not undergo the same scrutiny as federally overseen drug companies. The recent surge in compounding pharmacies’ production capabilities has coincided with an increase in unapproved peptide offerings, including **BPC-157**.
The trend has also drawn interest from health advocates like **Robert F. Kennedy Jr.**, who has actively promoted peptides within his **Make America Healthy Again** movement. Kennedy has vowed to challenge the FDA’s restrictive stance on peptides, which have garnered a substantial following among his supporters. Prominent marketers in the peptide space, including “biohacker” **Gary Brecka** and functional medicine physician **Dr. Mark Hyman**, have contributed to this growing popularity.
As the peptide trend continues to evolve, the FDA’s recent actions indicate heightened scrutiny. The agency has included more than two dozen peptides on a temporary list of substances deemed unsuitable for compounding due to safety concerns. The ongoing debate surrounding these unapproved peptides raises important questions about health, safety, and the influence of celebrity endorsements in the wellness industry.
-

 Politics1 week ago
Politics1 week agoSecwepemc First Nation Seeks Aboriginal Title Over Kamloops Area
-

 World4 months ago
World4 months agoScientists Unearth Ancient Antarctic Ice to Unlock Climate Secrets
-

 Entertainment4 months ago
Entertainment4 months agoTrump and McCormick to Announce $70 Billion Energy Investments
-

 Lifestyle4 months ago
Lifestyle4 months agoTransLink Launches Food Truck Program to Boost Revenue in Vancouver
-

 Science4 months ago
Science4 months agoFour Astronauts Return to Earth After International Space Station Mission
-

 Technology3 months ago
Technology3 months agoApple Notes Enhances Functionality with Markdown Support in macOS 26
-

 Top Stories1 month ago
Top Stories1 month agoUrgent Update: Fatal Crash on Highway 99 Claims Life of Pitt Meadows Man
-

 Sports4 months ago
Sports4 months agoSearch Underway for Missing Hunter Amid Hokkaido Bear Emergency
-

 Politics3 months ago
Politics3 months agoUkrainian Tennis Star Elina Svitolina Faces Death Threats Online
-

 Politics4 months ago
Politics4 months agoCarney Engages First Nations Leaders at Development Law Summit
-

 Technology4 months ago
Technology4 months agoFrosthaven Launches Early Access on July 31, 2025
-

 Top Stories3 weeks ago
Top Stories3 weeks agoFamily Remembers Beverley Rowbotham 25 Years After Murder