Health
FluidAI Secures FDA Clearance for Innovative Surgery Monitor
FluidAI Medical has achieved a significant milestone by obtaining clearance from the United States Food and Drug Administration (FDA) for its innovative device designed to predict post-surgery complications. The FDA granted the 510(k) clearance for FluidAI’s Origin product as a Class 2 medical device last month, providing the Kitchener-Waterloo-based health technology startup access to the substantial American healthcare market.
“This approval allows FluidAI to bring Origin to patients and hospitals in the world’s largest healthcare market,” said Youssef Helwa, co-founder and CEO of FluidAI. He emphasized the extensive effort and resources invested in reaching this point, noting that the company spent the last five years developing and refining the device while conducting studies across four continents. Helwa described the FDA clearance as a gateway for FluidAI to raise a Series B funding round to support its expansion into the U.S.
Founded in 2014 and previously known as Nerv Technology, FluidAI specializes in “data-driven surgical recovery.” The company focuses on predicting post-surgical complications by analyzing patient data and fluid. To date, FluidAI has concentrated its efforts primarily on general and gastrointestinal surgery. The company collaborates with over 35 medical centres, including prestigious institutions like Ohio’s Cleveland Clinic and Toronto’s University Health Network.
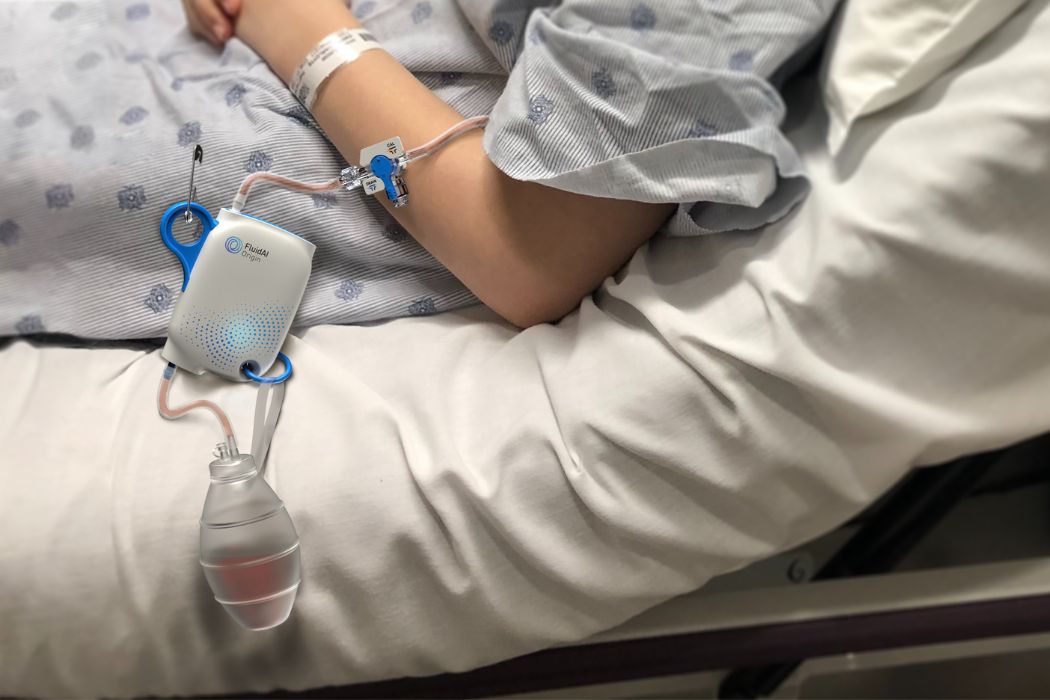
FluidAI’s Origin device is a real-time surgical drain monitor that analyzes the biochemical composition of patient fluid to identify potential complications early. By attaching to existing drains, Origin employs a combination of sensors and machine learning to measure factors like pH, electrical conductivity, and temperature. The device aims to detect potentially life-threatening anastomotic leaks before symptoms typically present, thereby improving patient outcomes.
Having previously marketed Origin in Canada and the Middle East, Helwa estimates that the device could support nearly 3.5 million patients in the U.S. alone. Origin is one of two core products from FluidAI; the second is the Stream Care software platform. This platform utilizes artificial intelligence to predict the risk of complications based on electronic health record information and data from devices like Origin.
Stream Care also enhances administrative efficiency by generating clinical documents and improving coding processes, thereby alleviating the burden on healthcare professionals. Additionally, it facilitates patient engagement and education through features such as a conversational assistant and symptom flagging. Stream Care is already operational in hospitals and clinics across Canada, the U.S., and the Middle East.
FluidAI recently closed a $15 million Series A funding round in 2023, bringing its total funding to just over $20 million. While Helwa did not disclose the current revenue, he confirmed that FluidAI has sufficient manufacturing capacity in the Kitchener-Waterloo area to meet its immediate and early U.S. expansion needs.
Despite challenges posed by U.S. tariffs, Helwa indicated that FluidAI has managed to mitigate the negative impact through strategic partnerships. As part of its growth strategy, the company plans to establish a U.S. office to better serve clients, partners, and patients. Whether there will be a need to build a manufacturing facility in the U.S. remains uncertain.
Looking ahead, FluidAI aims to support a total of 100,000 operations and expand its offerings to include a broader range of surgical types along with new product introductions. This latest FDA clearance positions FluidAI for substantial growth within the competitive landscape of healthcare technology, with the potential to significantly impact patient recovery outcomes.
-

 Politics4 weeks ago
Politics4 weeks agoSecwepemc First Nation Seeks Aboriginal Title Over Kamloops Area
-

 World5 months ago
World5 months agoScientists Unearth Ancient Antarctic Ice to Unlock Climate Secrets
-

 Entertainment5 months ago
Entertainment5 months agoTrump and McCormick to Announce $70 Billion Energy Investments
-

 Science5 months ago
Science5 months agoFour Astronauts Return to Earth After International Space Station Mission
-

 Lifestyle5 months ago
Lifestyle5 months agoTransLink Launches Food Truck Program to Boost Revenue in Vancouver
-

 Technology3 months ago
Technology3 months agoApple Notes Enhances Functionality with Markdown Support in macOS 26
-

 Lifestyle3 months ago
Lifestyle3 months agoManitoba’s Burger Champion Shines Again Amid Dining Innovations
-

 Top Stories2 months ago
Top Stories2 months agoUrgent Update: Fatal Crash on Highway 99 Claims Life of Pitt Meadows Man
-

 Politics4 months ago
Politics4 months agoUkrainian Tennis Star Elina Svitolina Faces Death Threats Online
-

 Sports5 months ago
Sports5 months agoSearch Underway for Missing Hunter Amid Hokkaido Bear Emergency
-

 Politics5 months ago
Politics5 months agoCarney Engages First Nations Leaders at Development Law Summit
-

 Technology5 months ago
Technology5 months agoFrosthaven Launches Early Access on July 31, 2025