Health
Gilead’s Trodelvy Fails Key Trial for First-Line Breast Cancer Treatment

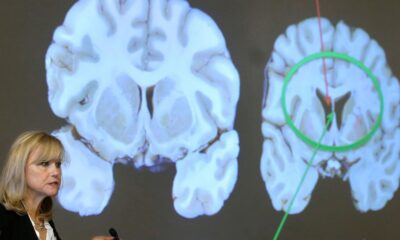
Gilead Sciences announced on March 15, 2024, that its Phase 3 clinical trial for the antibody-drug conjugate Trodelvy did not meet its primary endpoint. The trial aimed to evaluate Trodelvy as a first-line treatment for specific patients with breast cancer who had previously undergone treatment. This setback raises concerns regarding the drug’s potential role in a competitive market.
The trial was designed to assess the effectiveness of Trodelvy compared to standard therapies in patients with triple-negative breast cancer, a particularly aggressive form of the disease. Although Trodelvy is already approved by the U.S. Food and Drug Administration (FDA) for later-stage breast cancer, this trial sought to establish its efficacy in earlier stages of treatment.
In this extensive trial, researchers enrolled approximately 800 patients across multiple sites, aiming to provide robust data on the drug’s performance as a primary treatment option. Despite the initial optimism surrounding Trodelvy’s potential, the trial results indicate a significant hurdle for Gilead’s strategy in oncology.
Gilead’s Chief Medical Officer, Dr. Andrew Dickinson, expressed disappointment over the trial’s outcome. In a statement, he highlighted the ongoing commitment to exploring new treatment options for patients facing challenging diagnoses. The company plans to analyze the detailed data from the trial to understand the implications and potential next steps.
The failure of this trial could have financial repercussions for Gilead. As the pharmaceutical industry increasingly shifts towards innovative cancer therapies, maintaining a competitive edge is crucial. Gilead’s stock price may face pressure as investors reassess the company’s pipeline and future prospects in oncology.
Trodelvy, which has shown promise in treating patients with metastatic breast cancer after chemotherapy, has garnered attention for its unique mechanism that targets cancer cells while minimizing damage to healthy tissue. The drug’s prior success in later-stage treatments had set high expectations for its performance in earlier lines of therapy.
The broader implications of this trial failure extend beyond Gilead itself, as it reflects the challenges many companies face in developing effective treatments for complex cancers. The pharmaceutical landscape for oncology is highly competitive, with numerous entities vying to bring effective therapies to market.
As Gilead moves forward, it will need to reassess its development strategy and explore potential collaborations or alternative therapies. The company remains committed to advancing treatment options for patients with cancer, but the path ahead may require new approaches and innovations.
The results of this trial will likely provoke discussions within the medical community regarding treatment protocols for breast cancer. Gilead’s findings may influence future research directions and clinical practices as healthcare professionals continue to seek the most effective therapies for their patients.
-

 World4 months ago
World4 months agoScientists Unearth Ancient Antarctic Ice to Unlock Climate Secrets
-

 Entertainment4 months ago
Entertainment4 months agoTrump and McCormick to Announce $70 Billion Energy Investments
-

 Lifestyle4 months ago
Lifestyle4 months agoTransLink Launches Food Truck Program to Boost Revenue in Vancouver
-

 Science4 months ago
Science4 months agoFour Astronauts Return to Earth After International Space Station Mission
-

 Politics21 hours ago
Politics21 hours agoSecwepemc First Nation Seeks Aboriginal Title Over Kamloops Area
-

 Technology2 months ago
Technology2 months agoApple Notes Enhances Functionality with Markdown Support in macOS 26
-

 Top Stories4 weeks ago
Top Stories4 weeks agoUrgent Update: Fatal Crash on Highway 99 Claims Life of Pitt Meadows Man
-

 Sports4 months ago
Sports4 months agoSearch Underway for Missing Hunter Amid Hokkaido Bear Emergency
-

 Politics3 months ago
Politics3 months agoUkrainian Tennis Star Elina Svitolina Faces Death Threats Online
-

 Politics4 months ago
Politics4 months agoCarney Engages First Nations Leaders at Development Law Summit
-

 Technology4 months ago
Technology4 months agoFrosthaven Launches Early Access on July 31, 2025
-

 Top Stories2 weeks ago
Top Stories2 weeks agoFamily Remembers Beverley Rowbotham 25 Years After Murder