Health
Health Canada Issues Warning Against Unauthorized GLP-1 Drugs
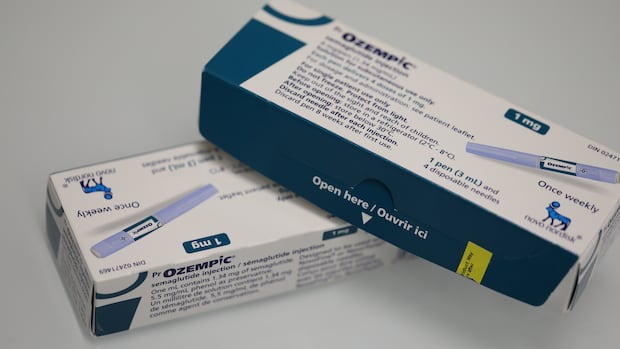
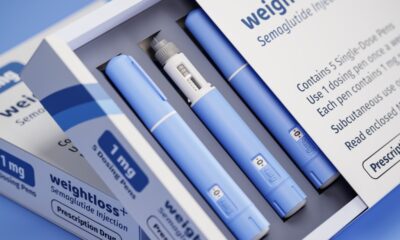
Health Canada has issued a strong warning against the purchase and use of unauthorized or counterfeit GLP-1 drugs, including the widely recognized semaglutide, an active ingredient found in Ozempic and Wegovy. In a public advisory released on October 25, 2023, the agency cautioned that the rising interest in GLP-1 medications for weight loss has led some consumers to seek out potentially harmful alternatives that have not undergone proper safety assessments.
The advisory highlights that only certain GLP-1 products have been authorized for use in Canada, specifically semaglutide, available in injection form as Ozempic and Wegovy, as well as Rybelsus oral tablets. Additionally, tirzepatide, marketed as Mounjaro and Zepbound injections, is also approved. Despite this, Health Canada has identified several retailers across the country selling unauthorized versions, often referred to as “fauxzempic,” through both physical stores and online platforms.
Risks Associated with Counterfeit Drugs
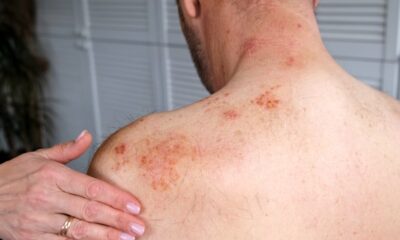
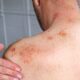
Health Canada emphasizes the significant risks associated with using unauthorized or counterfeit GLP-1 medications. These products may lead to severe health complications, including infections due to lack of sterility, allergic reactions, and other serious adverse effects resulting from contamination or improper handling. Health Canada has noted, “Selling unauthorized or counterfeit products or making false or misleading claims to prevent, treat or cure illnesses is illegal in Canada.”
The agency further reminded consumers that it does not endorse any health products and does not permit its logo to be used in advertising or packaging. Some misleading websites and advertisements have been found misusing official Health Canada logos, contributing to consumer confusion.
The regulator has also outlined known risks linked to genuine GLP-1 products. These include severe gastrointestinal issues, pancreatitis, worsening kidney injury, and low blood sugar, particularly when combined with other medications.
Proactive Measures and International Concerns
In response to the proliferation of unauthorized products, Health Canada is actively monitoring the marketplace. The agency can take action by seizing products and issuing compliance or warning letters regarding the sale and advertisement of these medications. Moreover, it is collaborating with the Canada Border Services Agency (CBSA) to intercept unauthorized shipments entering the country.
Concerns over counterfeit semaglutide products are not limited to Canada. In June 2024, the World Health Organization (WHO) reported instances of falsified semaglutide detected in countries including Brazil, the United Kingdom, and the United States the previous year. This underlines the global challenge of ensuring the safety and efficacy of medications, particularly as demand for weight loss drugs continues to rise.
As the situation develops, Health Canada remains committed to protecting public health by ensuring that only safe and effective medications are available to Canadians. Individuals seeking GLP-1 drugs are urged to consult with healthcare professionals and obtain prescriptions through legitimate sources.
-

 Politics3 months ago
Politics3 months agoSecwepemc First Nation Seeks Aboriginal Title Over Kamloops Area
-

 World7 months ago
World7 months agoScientists Unearth Ancient Antarctic Ice to Unlock Climate Secrets
-

 Top Stories1 month ago
Top Stories1 month agoUrgent Fire Erupts at Salvation Army on Christmas Evening
-

 Sports1 month ago
Sports1 month agoCanadian Curler E.J. Harnden Announces Retirement from Competition
-

 Lifestyle5 months ago
Lifestyle5 months agoManitoba’s Burger Champion Shines Again Amid Dining Innovations
-

 Top Stories2 months ago
Top Stories2 months agoFatal Crash on Highway 11 Claims Three Lives, Major Closure Ongoing
-

 Entertainment7 months ago
Entertainment7 months agoTrump and McCormick to Announce $70 Billion Energy Investments
-

 Science7 months ago
Science7 months agoFour Astronauts Return to Earth After International Space Station Mission
-

 Lifestyle7 months ago
Lifestyle7 months agoTransLink Launches Food Truck Program to Boost Revenue in Vancouver
-

 Technology5 months ago
Technology5 months agoApple Notes Enhances Functionality with Markdown Support in macOS 26
-

 Top Stories1 month ago
Top Stories1 month agoBlue Jays Sign Kazuma Okamoto: Impact on Bo Bichette’s Future
-

 Top Stories2 months ago
Top Stories2 months agoNHL Teams Inquire About Marc-André Fleury’s Potential Return