Science
Montreal’s AI Surgical Device Extends Lives of Cancer Patients
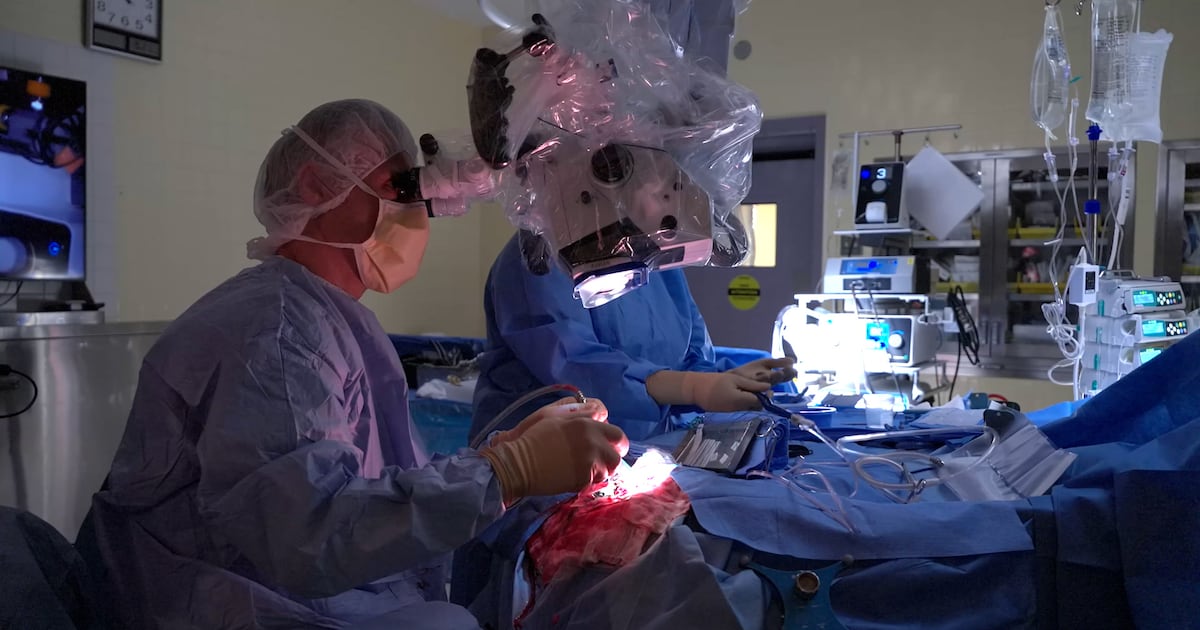
A groundbreaking surgical device developed in Montreal is changing the lives of cancer patients by leveraging artificial intelligence (AI) to improve surgical outcomes. Patients like Peter Ross, who was diagnosed with a brain tumor and given just 14 months to live nearly four years ago, are now finding hope and extended life thanks to this innovative technology.
Ross’s journey took a pivotal turn when he was treated by Dr. Kevin Petrecca at Montreal Neuro. Dr. Petrecca is the co-founder of SENTRY, a handheld device designed to differentiate between cancerous and healthy tissues during surgery. “It’s given me, the rest of my life,” Ross expressed. His wife, Sandrine Menard, echoed his gratitude, stating, “Whatever happens, we’re grateful.”
The SENTRY device boasts an impressive accuracy rate. According to Dr. Petrecca, it identifies tumors with a reliability of 98.7 percent. More importantly, when it indicates that brain tissue is normal, it is correct 100 percent of the time. This level of precision allows surgeons to excise more cancerous cells than previously possible, addressing the challenge of tumors that are not visible to the naked eye or other technologies.
Dr. Petrecca explains how the device works: “You make contact with the spot, and you will get feedback within less than three seconds if it contains tumor cells or not.” The implications of using this device during surgery are profound; it can potentially extend a patient’s life by two to five times longer than expected. While it has primarily been tested in brain surgeries, the technology is also applicable for detecting cancer in other organs, such as the breast and lungs.
Hundreds of patients have participated in clinical trials involving SENTRY, including Ross. He reflects on the additional time he has gained: “I take every extra day that’s given to me to go out and walk, and just, you know, enjoy life because I owe that to this surgery.”
Looking ahead, the next critical step for SENTRY is obtaining approval from the FDA. A pivotal trial is scheduled for May 2024, and Ross is hopeful that this medical advancement will continue to help more patients reach significant life milestones.
As the landscape of cancer treatment evolves with innovations like SENTRY, the stories of individuals like Peter Ross remind us of the profound impact that technology can have on human life.
-

 Politics4 weeks ago
Politics4 weeks agoSecwepemc First Nation Seeks Aboriginal Title Over Kamloops Area
-

 World5 months ago
World5 months agoScientists Unearth Ancient Antarctic Ice to Unlock Climate Secrets
-

 Entertainment5 months ago
Entertainment5 months agoTrump and McCormick to Announce $70 Billion Energy Investments
-

 Science5 months ago
Science5 months agoFour Astronauts Return to Earth After International Space Station Mission
-

 Lifestyle5 months ago
Lifestyle5 months agoTransLink Launches Food Truck Program to Boost Revenue in Vancouver
-

 Technology3 months ago
Technology3 months agoApple Notes Enhances Functionality with Markdown Support in macOS 26
-

 Lifestyle3 months ago
Lifestyle3 months agoManitoba’s Burger Champion Shines Again Amid Dining Innovations
-

 Top Stories2 months ago
Top Stories2 months agoUrgent Update: Fatal Crash on Highway 99 Claims Life of Pitt Meadows Man
-

 Politics4 months ago
Politics4 months agoUkrainian Tennis Star Elina Svitolina Faces Death Threats Online
-

 Sports5 months ago
Sports5 months agoSearch Underway for Missing Hunter Amid Hokkaido Bear Emergency
-

 Politics5 months ago
Politics5 months agoCarney Engages First Nations Leaders at Development Law Summit
-

 Technology5 months ago
Technology5 months agoFrosthaven Launches Early Access on July 31, 2025





















