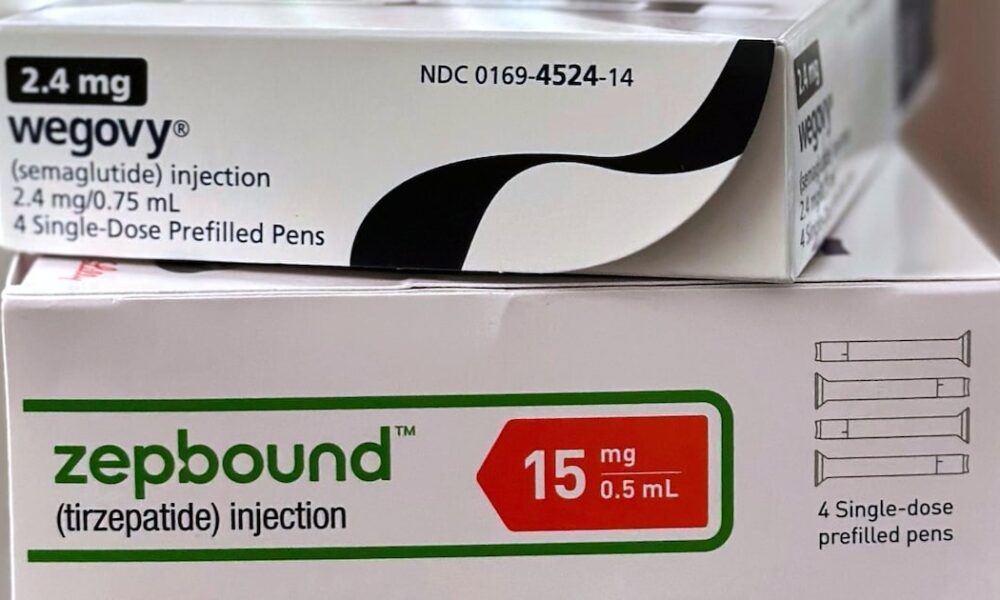

Eli Lilly has announced a reduction in the price of its obesity treatment, Zepbound, to enhance affordability for U.S. patients. Starting immediately, patients with a prescription...



The United States Food and Drug Administration (FDA) has announced a brief extension to its re-review of the partial clinical hold on Vanda Pharmaceuticals’ investigational drug,...



Understanding the process of selecting candidates for clinical research trials is crucial for ensuring participant safety and ethical standards. Clinical trials are governed by strict regulations...



The introduction of cloned meats into international markets has sparked significant concern regarding their quality and the potential impact on traditional farmers. As cloned products gain...
OS Therapies Inc. (NYSE American: OSTX), a leader in listeria-based cancer immunotherapies, announced its third quarter 2025 financial results and provided a comprehensive business update. The...
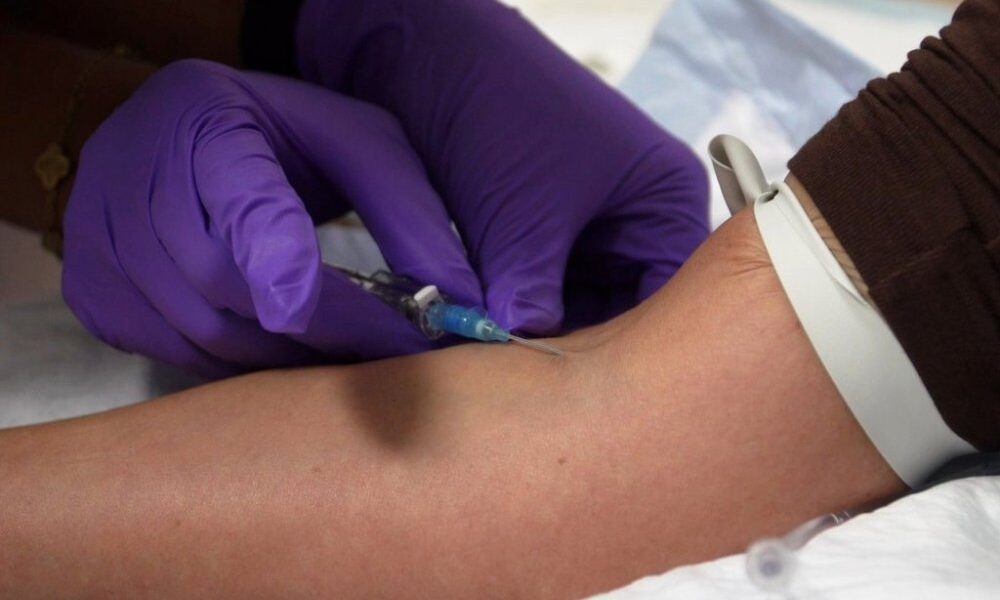
Unapproved peptide injections have surged in popularity, championed by wellness influencers, fitness coaches, and celebrities as a means to enhance muscle growth, promote weight loss, and...
Johnson & Johnson (J&J) has submitted a new application to the U.S. Food and Drug Administration (FDA) to expand the use of its medication, Stelara (ustekinumab),...

Akebia Therapeutics has officially halted its plans to expand the indication for its drug Vafseo (vadadustat), aimed at treating anemia in patients with chronic kidney disease...



The U.S. Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) to Elanco Animal Health for its product Credelio (lotilaner), designed to treat infections...



Novo Nordisk has secured approval from the U.S. Food and Drug Administration (FDA) to market its oral GLP-1 medication, Rybelsus, specifically aimed at reducing the risk...